Ashley Vij, USAID
Emily Dorward, USAID
Messai Belayneh, USAID
Anita Dam, USAID
This post was first published on USAID’s website.
Over the last 20 years, USAID has been a leader with partner countries in offering comprehensive HIV prevention, treatment, and care services helping to turn the tide on HIV.
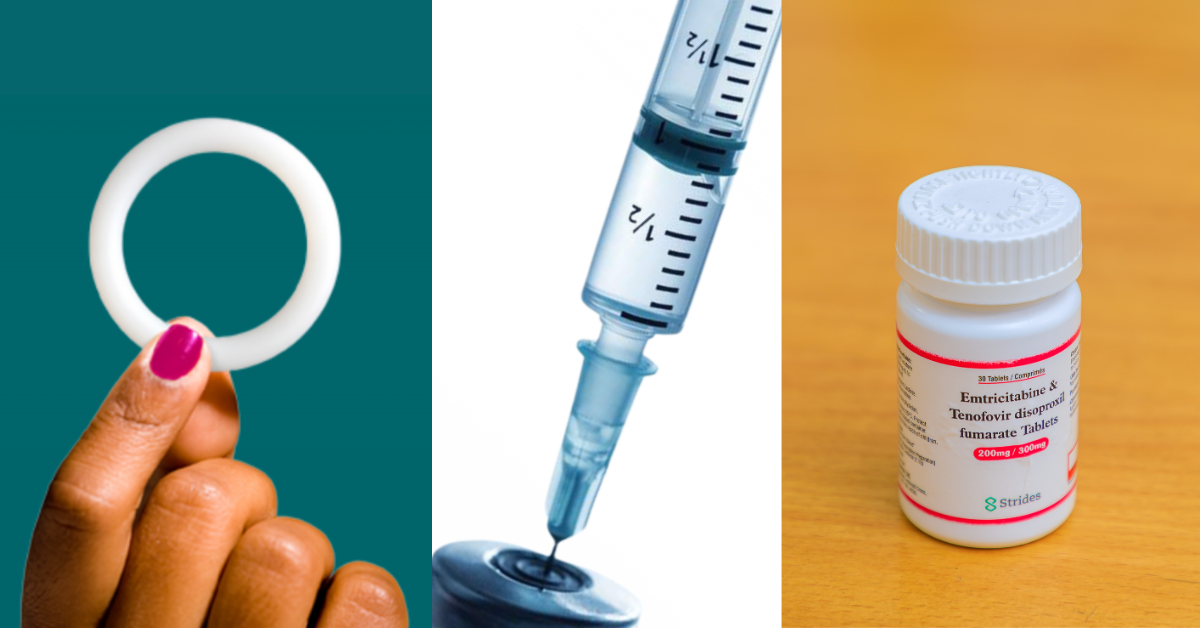
Through oral pre-exposure prophylaxis (PrEP), condoms, voluntary medical male circumcision, and antiretroviral treatment, USAID has helped to expand prevention options and access for all. Today, USAID continues to provide global leadership in the HIV response by expanding access to options with a new generation of HIV prevention products. The PrEP ring, also known as the dapivirine ring, is a silicone vaginal ring that slowly releases dapivirine, an antiretroviral drug, over the course of a month. CAB PrEP, also known as the long-acting injectable cabotegravir, is a bi-monthly intramuscular injection.
In 2021, PEPFAR through USAID began investments in the Maximizing Options to Advance Informed Choice for HIV Prevention (MOSAIC) project, led by FHI360 and local partners, known as the MOSAIC Consortium, to accelerate the introduction and scale-up of new HIV prevention products. This year, through the MOSAIC Consortium, USAID launched the Catalyzing Access to New Prevention Products to Stop HIV (CATALYST) study, the largest and first implementation science study of its kind to provide oral PrEP, CAB PrEP, and the PrEP ring to women. All three PrEP products will be available at 28 PEPFAR service delivery sites in Kenya, Lesotho, South Africa, Uganda, and Zimbabwe through CATALYST.

In order to bridge the gap between clinical trial research and large-scale implementation of HIV prevention products, we must understand how to optimally deliver these products in a real-world setting. Building on lessons from informed choice in voluntary family planning and the implementation of oral PrEP, implementation science studies will help us to build an evidence base for the introduction of this new generation of HIV prevention products. This targeted, multi-country implementation study will provide the much-needed context to understand how to:
- operationalize HIV prevention product choice within health systems and service delivery programs;
- identify product preferences and patterns of use among different population groups; and,
- examine barriers and enablers to the uptake and adherence of these new prevention products.
The findings and insights from CATALYST will help increase the precision of HIV prevention programming – ultimately saving more lives.
CATALYST offers a critical opportunity to better understand the demand for the different PrEP methods when offered as a choice. Data from CATALYST will help estimate the need of these new PrEP products in the medium to long term, which will inform and provide visibility for generic manufacturers to make new products even more affordable. Better demand visibility will improve supply chain planning for all PrEP methods.
To help more providers effectively counsel patients on the three PrEP options in a stigma-free environment, CATALYST has developed provider training tools and job aids, and has embedded trainers for providers on how to counsel patients on each PrEP method. This training and experience equip providers with the skills to not only discuss these three PrEP methods, but also better prepare providers to counsel on choice within an HIV prevention product platform for the future.
PEPFAR and USAID investments in the MOSAIC Consortium and the CATALYST study will help create a sustainable, enabling environment to introduce new HIV prevention products. CATALYST will demonstrate the provision of these new PrEP products in existing partner country health systems, strengthening these systems, from supply chain to provider training to access for users, and all parts in between. USAID will translate the lessons learned from CATALYST globally into programs, using the quick turnaround on data sharing and near-real time transfer of best practices to expedite the time required to move from product introduction to implementation. The outcomes from this study will improve access, counseling, and choice for women as this new generation of HIV prevention products comes to market.