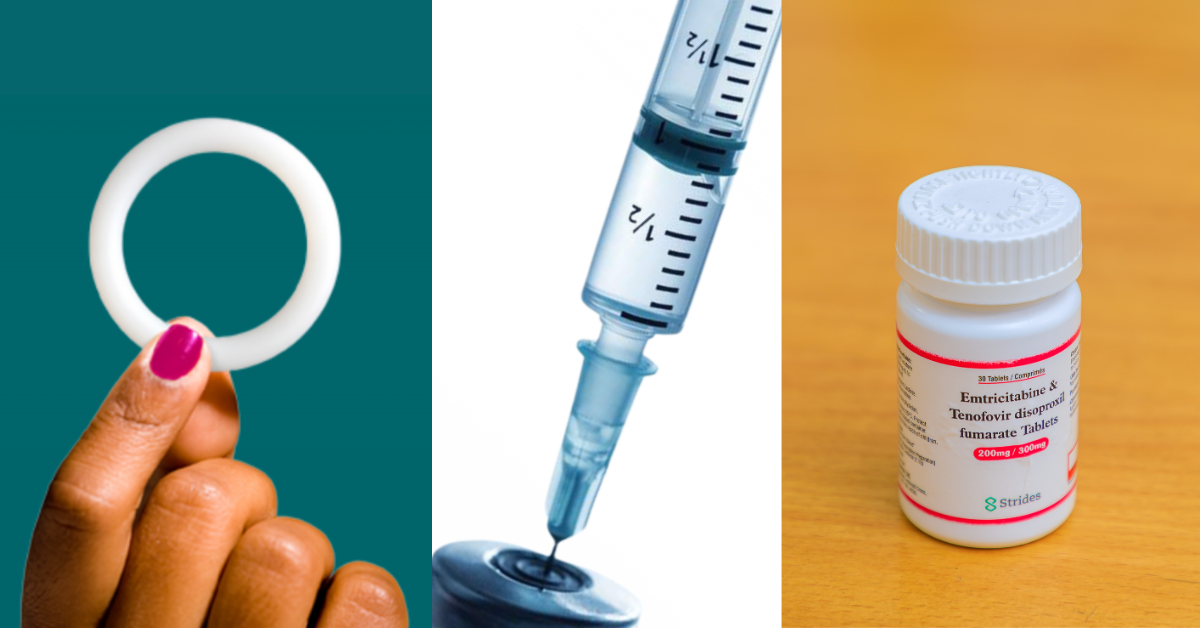
When a country is poised to introduce a new biomedical prevention product, policymakers want to ensure that their rollout plan is based on the latest science and provides clear, standardized procedures for implementation. The WHO publishes global guidance on delivering new HIV prevention products, but those recommendations need to be adapted to the local context.
Continue Reading